ABSTRACT By visualizing DNA with diamidino
phenylindole (DAPI), we found that hypothermal incubation followed by rewarming
of human neutrophils resulted in an increased number of DAPI-positive objects
representative of extensive DNA unfolding seemingly similar to neutrophil
extracellular traps (NETs). In contrast to canonical NET formation, diphenylene
iodonium (DPI), an NADPH oxidase inhibitor, exhibited negligible effects on
formation of the DAPI-positive objects. Moreover, multiple instances of DNA
damage were detected in the objects, but not in canonical NETs. Our results thus
suggest the potential of hypothermia for triggering DNA structural alteration
in neutrophils, which is similar to but distinct from NET formation. Keywords:
Hypothermal Treatment; DNA Unfolding; Neutrophil Extracellular Trap (NET) 1.
Introduction Low-temperature conditions, referred to as hypothermia, are
generally used for the storage of cells, tissues, organs and bodies for both
scientific and clinical purposes. Hypothermia is an important means of slowing
down cellular metabolism during storage, thus inhibiting injurious processes
caused by the deficiency of oxygen and substrate supply. However, hypothermia
can give rise to cell injury, including cell death [1,2]. Neutrophils are a
main type of effector cell in the innate immune system [3,4], which circulate
in the blood and engulf invading microorganisms such as bacteria and fungi by
phagocytosis. In addition to such activities, Brinkmann et al. have reported
that, following activation by microorganisms, neutrophils can undergo
morphological changes detectable by microscopic observations [5]. These changes
include loss of the lobular-shaped nucleus followed by disintegration of the
nuclear envelope, which allow nuclear, cytoplasmic and granular components to
mix together and subsequently rupture the cell membrane to release the
DNA/chromatin into the extracellular environment [5]. The result is that the
unfolded DNA/chromatin fibers with attached bactericidal proteins can function
as neutrophil extracellular traps (NETs) for microorganisms. NETs appear to be
the result of a unique form of cell death. Therefore, as opposed to apoptosis
and necrosis, Steinberg and Grinstein coined the term “NETosis” for neutrophil
cell death, which leads to the formation of NETs [6]. In addition to
microorganism infection, several physiological inducers of NETs have been
reported [7 and references herein]. For instance, platelets activated via
Tolllike receptor 4 rapidly induce NET formation [8]. Antibodies [9],
antibody-antigen complexes [10,11], human immunodeficiency virus (HIV-1) [12],
and microbial components such as lipopolysaccharide [13,14] are also known to
induce the formation of NETs. Although the intracellular signaling pathway(s)
that transmit these physiological stimuli remain largely unknown, reactive
oxygen species (ROS) generation was demonstrated to be an absolute requirement
for NET formation [15,16]. Thus, one of the most widely-used agents to induce
NETs in in vitro experiments is phorbol-12-myristate-13-acetate (PMA), which
directly stimulates protein kinase C (PKC) leading to potent activation of
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which in turn
generates superoxide [5,7,17]. Therefore, it is reasonable to use diphenylene
iodonium (DPI), a NADPH oxidase inhibitor [18], to block the formation of
PMA-stimulated NETs [15,17,19]. In this study, we found that hypothermal
incubation of * Corresponding author. Copyright © 2013 SciRes. CellBio 118 J.
KAWATA ET AL. human neutrophils at 4˚C for up to 1 h followed by incubation at
37˚C resulted in an increased number of DAPI-stainable objects similar to
global DNA unfolding observed in PMA-stimulated NETs. However, our additional
experimental data revealed that hypothermia/rewarming-induced DNA unfolding was
regulated in a manner similar to, but biochemically and pharmacologically
distinct from, canonical NETs. Although the molecular mechanism of this
phenomenon is not fully understood, we inferred, based on our experimental
data, the possible role of ROS, which were generated during hypothermia/rewarming-treatment
in a manner independent of NADPH oxidase activity in the formation of the
DAPI-positive, NET-like objects. 2. Materials and Methods 2.1. Peripheral Blood
Preparation and Culture Human peripheral blood preparations (from two normal
male donors, collected in compliance with Kumamoto Health Science University
and approved by the University Oversight Committee) were enriched for
neutrophils by density gradient centrifugation with HISTOPPAQUE 117
(Sigma-Aldrich) and Lymphocyte Separation Solution 1.119 (Nakarai Tesque)
according to the procedures described by the supplier. Washed enriched
neutrophilic fractions were counted and examined for purity using Wright Giemsa
staining (Sigma-Aldrich). 2.2. Drug and Hypothermal Treatments Cells were incubated
in culture dishes containing an immersed coverslip in RPMI 1640 (Sigma-Aldrich)
supplemented with 5% fetal bovine serum (FBS), 1% penicillin/streptomycin and
0.1% gentamaycin in a humidified atmosphere containing 5% CO2. To induce NETs,
PMA (Wako Pure Chemical Industries) was added to the culture medium at a
concentration of 50 nM and incubated for 4 h at 37˚C. To inhibit NADPH oxidase
activity, DPI (Cayman Chemical) was added to the culture medium at a
concentration of 20 μM. Hypothermal treatment and rewarming of cells were
performed by incubation in a humidified atmosphere containing 5% CO2. After
drug and/or hypothermal/rewarming treatment, the coverslips were removed from
the cultures and subjected to appropriate assays. DNA was visualized by staining
with DAPI. 2.3. Antibodies and Immunostaining Cells were washed once for 5 min
with ice-cold PBS and then fixed with 4% paraformaldehyde in PBS for 5 min at
room temperature. After fixation, the cells were rinsed once with PBS and
subjected to indirect-immunofluorescence analysis using anti-neutrophil
elastase (Calbiochem), anti-histone H3 (Santa Cruz Biotechnology), and
anti-histone H3 citrulline R26 (Abcam) antibodies. The secondary antibodies
were obtained from Santa Cruz Biotechnology and Sigma-Aldrich. 2.4. Bacteria
Trapping Assay Escherichia coli BL21 (DE3) were transformed with pET28-EGFP, a
plasmid for expression of green fluorescent protein (GFP), and cultured in
Luria-Bertani (LB) medium containing kanamycin at 37˚C for 16 h. 107 E. coli cells
were incubated with a coverslip containing hypothermia/rewarming-induced
DAPI-positive objects in RPMI 1640 supplemented with 5% FBS at 37˚C. After 20
min at room temperature, the coverslips were washed three times with PBS
followed by incubation with 4% paraformaldehyde. DNA fibers were stained with
DAPI. Because the E. coli expressed GFP, bacteria trapped by DNA fibers could
be detected by fluorescence microscopy. For DNase I treatment, the coverslips
were treated with PBS containing 100 U/ml DNase I (Takara) at 37o C for 1 hr.
The numbers of E. coli with GFP signals on the coverslips were counted by
fluorescent microscopy. 2.5. TUNEL Assay Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assays were performed using the MEBSTAIN Apoptosis
TUNEL Kit II (MBL) according to the manufacturer’s instructions. The
TUNEL-positive cells were counted under a microscope. The percentage of
TUNEL-positive cells was defined by the number of positive cells among the
total number of cells in each sample. For one experiment, cells were counted in
at least three different microscopic fields of view. 2.6. Intracellular ROS
Detection Assay Intracellular ROS production was monitored using the cell
permeable fluorescent dye, CellROX Deep Red Reagent (Invitrogen). This agent
can readily react with ROS to form a fluorescent product proportional to the
amount of ROS generated in the cells. The cells were incubated with 5 μM
CellROX Deep Red for 30 min and then harvested. The fluorescence intensity of
the cells was measured using a FACSVerse flow cytometer (BD Biosciences). 2.7.
MitoTracker Analysis After fixation with 4% paraformaldehyde, cultured human
neutrophils were stained with the mitochondrionspecific dye, MitoTracker Red
CMXRRos (Invitrogen), according to the manufacturer’s instruction. The cells
were immediately analyzed using a FACSVerse flow cytometer. Copyright © 2013
SciRes. CellBio J. KAWATA ET AL. Copyright © 2013 SciRes. CellBio 119 2.8.
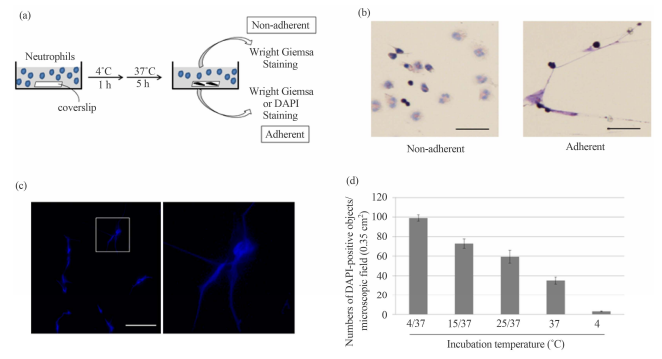
Statistical Analysis Wright–Giemsa staining (Figure 1(b), left panel). In
contrast, we unexpectedly found that small, but substantial, numbers of
Wright-Giemsa-stainable materials, which looked different from intact
neutrophils, were present on the coverslip (Figure 1(b), right panel). Unless
otherwise stated, all data are presented as the mean ± SD. Within individual
experiments, data points were based on a minimum of triplicate representative
samples and experiments were repeated at least three times. When the materials
on the coverslip were stained with DAPI without any fixative treatment, we
observed bright fluorescent signals under fluorescent microscopy, many of which
appeared to consist of multiple DAPI-positive strings (Figure 1(c)). Because
DAPI is a fluorescent dye that intercalates into double-stranded DNA, and that
living neutrophils are less permeable to the dye than dead neutrophils, we
thought it probable that these bright DAPI-stained signals represented global
DNA unfolding of dead neutrophils, which somehow adhered to the coverslip. It
should be mentioned that there were few neutrophils with normal morphology on
the coverslip per view field, implying that most of neutrophils floated in the
culture medium under the standard culture conditions. 3. Results and Discussion
3.1. Effect of Hypothermia on Human Neutrophils in Culture After isolating
human neutrophils from peripheral blood preparations (see Materials and
Methods), the cells (4.5 × 106 cells) were incubated at 4˚C for 1 h followed by
incubation at 37˚C for 5 h in the 6-cm culture dish supplemented with 2 ml of
the culture medium, in which a coverslip was immersed (Figure 1(a)). We found
that most of the cells were present as non-adherent forms, and were thus
floating in the culture medium. These non-adherent cells appeared
morphologically intact as shown by Figure 1. DAPI-stained objects in the
hypothermia/rewarming-treated human neutrophils. (a) Schematic representation
of procedure for detecting the hypothermia/rewarming-induced DAPI-positive
objects. Human neutrophils from peripheral blood preparations were cultured in
dishes containing a coverslip at 4˚C for 1 h followed by rewarming at 37˚C for
5 h. Non-adherent and adherent materials in the culture medium stained with
Wright Giemsa; (b) Non-adherent and adherent materials in the culture were stained
with Wright Giemsa (left and right panels). Bar indicates 50 μm; (c) The
morphologies of the DAPI-stained objects adherent to the coverslip were
detected by fluorescence microscopy (left panel). Bar indicates 50 μm. The
panel on the right shows a higher magnification of the region indicated in the
left panel; (d) Human neutrophils from peripheral blood preparations (1 × 106
cells) were cultured in dishes containing a coverslip for 6 h at 4˚C (4), at
4˚C for 1 h followed by rewarming at 37˚C for 5 h (4/37), at 15°C for 1 h
followed by rewarming at 37˚C for 5 h (15/37), at 25˚C for 1 h followed by
rewarming at 37˚C for 5 h (25/37), and at 37˚C for 6 h (37). After incubation
under the conditions as indicated, the numbers of DAPI-positive objects on the coverslips
in the microscopic field (0.35 cm2 ) were counted. The values shown represent
means ± SE of three independent experiments. 120 J. KAWATA ET AL. We then
investigated whether the requirement for DAPI-positive object production was
simple exposure to hypothermia or rather the combination of hypothermia/
rewarming. When human neutrophils were maintained at a constant temperature of
either 4˚C or 37˚C, significantly less DAPI-positive signals were detected as
compared with cells cultured either at 4˚C, 15˚C, or 25˚C for 1 h followed by
incubation at 37˚C for 5 h (Figure 1(d)). These results suggest that the
appearance of the DAPIpositive objects was associated with incubation of
neutrophils under hypothermal conditions followed by rewarming. 3.2. Comparison
of the Biochemical and Immunohistochemical Properties of
Hypothermia/Rewarming-Induced DAPI-Positive Objects and PMA-Stimulated NETs
When we observed the DAPI-positive objects in hypothermia/rewarming-treated
human neutrophils, we noticed that morphological similarities between the
objects and DAPI-stained PMA-stimulated NETs, leading us to suspect that the
DAPI-positive objects per se might represent NETs (Figure 2(a)). To investigate
this possibility, we first asked whether the that DAPI-positive objects
possessed the ability to bind bacteria. Given NETs are defined as extracellular
DNA-proteinaceous structures that exhibit the ability to associate with a wide
variety of Gram-positive and Gram-negative pathogens [7], we expected that the
DAPI-positive structures might also show similar properties. As shown in Figure
2(b), when GFP-expressing E. coli was incubated with the coverslip containing
DAPI-positive objects, we found multiple GFP signals present together with the
DAPI-signals. Their ability to trap bacteria appeared equivalent to that of
PMA-stimulated NETs, because the number and distribution of GFP-signals
associated with the DAPI-positive objects were very similar to those associated
with PMA-stimulated NETs, suggesting that the objects possessed the ability to
trap bacteria. It should be mentioned that the number of bacteria trapped to
the DAPI-positive objects was reduced when the coverslips were treated with
DNase I (Figure 2(c)). Similar results were obtained when PMA-stimulated NETs
were treated with DNase I. These results imply that both structures are equally
susceptible to DNase I treatment with respect to bacterial trap. To further
evaluate the similarities between the DAPIpositive objects and canonical NETs,
we performed indirect-immunofluorescence analysis using antibodies that
recognize marker proteins for NETs: anti-neutrophil Figure 2.
Hypothermia/rewarming-induced DAPI-positive objects exhibited several features
similar to PMA-stimulated NETs. (a) Human neutrophils were incubated in a
culture dish containing a coverslip at 4˚C for 1 h followed by incubation at
37˚C for 5 h. The coverslip was removed and fixed in PBS containing 4%
paraformaldehyde and then stained with DAPI (left). For the control,
PMA-stimulated neutrophils, which exhibit canonical NETs, were fixed with 4%
paraformaldehyde and subjected to DAPI-staining (right). Bar indicates 50 μm;
(b) Human neutrophils were incubated in a culture dish containing a coverslip
at 4˚C for 1 h followed by incubation at 37˚C for 5 h. The coverslip was
removed and then transferred to culture medium containing E. coli expressing
recombinant GFP, followed by incubation for 15 min at 37˚C (left). For the
control, PMA-stimulated neutrophils were treated in the same way (right). The
arrows indicate GFP-signals that represent E. coli. Bar indicates 50 μm; (c)
After hypothermia/rewarming-(left) or PMA-incubation (right), the coverslips
were treated with DNase buffer alone (gray bars) or DNase buffer containing 100
U/ml DNase I (black bars) at 37˚C for 1 hr. The numbers of E. coli with GFP
signals on the coverslips in the microscopic field (0.35 cm2 ) were counted.
The values shown represent means ± SE of three independent experiments; (d) The
hypothermia/rewarming-induced DAPI-positive objects and PMAstimulated NETs were
immunostained with (upper panels in left and middle-right columns) or without
(upper panels in middle-right and right columns) anti-NE antibodies.
DAPI-stained images of each treatment are shown at the bottom. Bar indicates 50
μm; (e) The hypothermia/rewarming-induced DAPI-positive objects and
PMA-stimulated NETs were immunostained with (upper panels in left and
middle-right columns) or without (upper panels in middle-right and right
columns) anti-histone H3 antibodies. DAPI-stained images of each treatment are
shown at the bottom. Bar indicates 50 μm. Copyright © 2013 SciRes. CellBio J.
KAWATA ET AL. 121 elastase (NE) and anti-histone H3 antibodies [7]. As shown in
Figures 2(d) and (e), in the presence of these anti-bodies, the signals were
detected not only on PMAstimulated NETs but also on the DAPI-positive objects.
In contrast, in the absence of the antibodies, these signals were barely
detected, suggesting existence of NE and histone H3 on both the DAPI-positive
objects and canonical NETs. Taken together, at least with regard to their
ability to trap bacteria and the existence of two marker proteins for NETs, our
results indicated that the DAPI-positive objects possessed biochemical
similarities to canonical NETs. It should be noted, however, the experiments
described above are not sufficient to conclude that the DAPI-positive objects
have anti-bacterial activity. We are therefore investigating whether NE and
histones on the DAPIpositive objects can indeed inactivate bacterial toxic proteins,
called “virulence factors,” and inhibit bacterial growth. In addition, we wish
to test whether the DAPIpositive objects can capture microorganisms besides
Gram-negative bacteria (E. coli), such as fungi and parasites. 3.3. The
DAPI-Positive Objects Exhibited Several Biochemical Properties Different from
Those of PMA-Stimulated NETs Although our results so far indicated a
correlation between DAPI-positive objects and NETs, several differences were
also revealed. For instance, when indirect immunofluorescence analysis was
conducted using antihistone H3 citrulline R26 (anti-H3cit) antibody, we found
that the antibody stained many, but not all, PMAstimulated NETs, whereas the
antibody proved poor at detecting the DAPI-positive objects (Figure 3(a)). Because
it has been reported that peptidylarginine deiminase 4 (PAD4/PADI4), which
catalyzes histone hy percitrullination, mediates chromatin decondensation and
is vital to NET formation [13,20,21], our observation of different staining
patterns with anti-H3cit antibody between the DAPI-positive objects and
PMA-stimulated NETs implies activation of PAD4 in PMA-stimulated cells, while
the enzyme might not be fully activated in the hypothermia/rewarming-treated
cells. We also detected differences between the objects and NETs in TUNEL
assays. As shown in Figure 3(b), TUNEL-positive signals were negligible in
PMA-treated cells, confirming the previous report that no TUNELpositive DNA
damage is detectable in canonical NETs [15]. In contrast, TUNEL assay visualized
more than 90% of the hypothermia/rewarming-induced DAPI-positive objects,
indicating the existence of multiple sites of TUNEL-positive DNA damage on
extensively unfolded DNAs in the objects. Because TUNEL-positive signals are
frequently associated with apoptotic cells, these data indicated that
hypothermia/rewarming-incubation might trigger, to some extent, activation of
apoptosis-related DNA cleavage enzyme(s) in neutrophils, suggesting the
possible contribution of apoptotic signaling pathways, at least in part, to DNA
structural alterations during the formation of the DAPI-positive, NET-like
structures. 3.4. Involvement of ROS Elevation to Generate the DAPI-Positive
Objects Given that ROS generation is an absolute requirement for the formation
of NETs [15,16], we next assessed whether hypothermia/rewarming of neutrophils
coincided with the generation of ROS. Thus, we measured ROS in
hypothermia/rewarming-treated human neutrophils. As shown in Figure 4(a),
during constant temperature incubation at either 4˚C or 37˚C, no
increase/decrease in ROS was detected. In contrast, we found a significant
elevation of ROS when cells were kept at 4˚C followed (a) (b) Figure 3.
Hypothermia/rewarming-induced DAPI-positive, NET-like structures exhibited
several features distinct from PMA-stimulated NETs. (a) The
hypothermia/rewarming-induced DAPI-positive objects and PMA-stimulated NETs
were immunostained with (upper panels in left and middle-right columns) or
without (upper panels in middle-right and right columns) anti-H3cit antibodies.
DAPI-stained images of each treatment are shown at the bottom. Bar indicates 50
μm; (b) Hypothermia/rewarming-induced DAPI-positive, NET-like structures (left
column) and PMA-stimulated NETs (right column) were subjected to TUNEL assay
(upper panel). DNA was visualized with propidium iodide (PI; lower panel). Bar
indicates 50 μm. Copyright © 2013 SciRes. CellBio 122 J. KAWATA ET AL. (a) (b)
(c) Figure 4. Hypothermia/rewarming-induced DAPI-positive, NET-like structures
are biochemically and pharmacologically distinct from PMA-stimulated NETs. (a)
Human neutrophils were incubated at 4˚C for 1 h followed by incubation at 37˚C
for 1 h (indicated as “1/1”) or 3 h (indicated as “1/3”) in culture medium with
(+; black bars) or without (−; gray bars) 20 μM DPI. ROS formation by cells
incubated at 4˚C and 37˚C was quantified using CellROX Deep Red Reagent and
fold ROS generation (ROS formation at 37˚C over that at 4˚C) was calculated.
For the control, ROS formation was quantified during cell culture at 4˚C for 1
h (indicated as “1/0”) and 37˚C for 1 h or 4 h (indicated as “0/1” or “0/4”,
respectively) in the presence or absence of DPI, and fold ROS generation during
each culture period (ROS formation at the start over that at the end of
culture) was calculated; (b) Human neutrophils (1 × 106 cells) were incubated
at 4˚C for 1 h followed by incubation at 37˚C for 5 h in culture medium with
(black bars) or without (gray bars) 20 μM DPI. The numbers of DAPI-positive
objects on the coverslips in the microscopic field (0.35 cm2 ), which were in
proportion to total numbers of the DAPI-positive objects in the cultures, were
counted. For the control, human neutrophils were incubated at 37˚C in the
presence (+) or absence (−) of 20 μM DPI or 50 nM PMA and the numbers of NETs
were counted; (c) Human neutrophils were incubated at 4˚C for 1 h followed by
incubation at 37˚C for 1 h (indicated as “1/1”) or 3 h (indicated as “1/3”) in
culture medium with (+; black bars) or without (−; gray bars) 20 μM DPI. Cells
were subjected to MitoTracker Red-staining and fold changes in fluorescent
signals (signal at 37˚C over that at 4˚C) were calculated. For the control,
fluorescent signals were quantified during cell culture at 4˚C for 1 h
(indicated as “1/0”) and 37˚C for 1 h or 4 h (indicated as “0/1” or “0/4”,
respectively) in the presence or absence of DPI, and fold fluorescent signal
changes during each culture period (signal at the start over that at the end of
culture) were calculated. by incubation at 37˚C for 1 h or 3 h; an approximate
10- fold increase in ROS was apparent in the cells after incubation at 37˚C. We
next examined whether NADPH oxidase contributed to ROS production in
hypothermia/rewarmingtreated cells. Given that PMA-induced NET formation is
effectively inhibited by DPI, an inhibitor of NADPH oxidase activity [15,17,18
and see Figure 4(b)], we tested the effect of this drug. Intriguingly, neither
ROS generation nor the formation of DAPI-positive objects was significantly
affected by administration of DPI (Figures 4(a) and (b)), indicating that the
mechanism of ROS generation in hypothermia/rewarming-treated neutrophils could
be pharmacologically distinguished from that in PMA-stimulated cells with
respect to the involvement of NADPH oxidase in ROS generation. Copyright © 2013
SciRes. CellBio J. KAWATA ET AL. 123 Although where and how ROS are produced in
the hypothermia/rewarming-treated cells remains unclear, it is noteworthy that
the mitochondrion-specific dye, MitoTracker Red, detected structural and/or
functional alterations in mitochondria in the hypothermia/rewarmingtreated
cells (Figure 4(c)). Because mitochondria are known to produce ROS in aerobic
organisms, including humans [22], these data suggest scenario that this
organelle may generate ROS during hypothermia/rewarming, leading to the
formation of the DAPI-positive, NET-like structures. However, DPI is also known
as a potent inhibitor of mitochondrial reactive oxygen species production [23].
If DPI inhibits generation of ROS from both mitochondria and the NADPH oxidase
pathway during hypothermia/rewarming of neutrophils, we need to consider the
possibility of an alternative pathway to generate ROS, besides the NADPH
oxidase pathway or the mitochondria pathway. 4. Conclusion DAPI-positive
objects with extensive DNA unfolding were observed in human neutrophils
cultured in hypothermic conditions followed by rewarming. Our experimental data
indicated that such DNA structural alterations in neutrophils may be related to
NET formation, but can be biochemically and pharmacologically discriminated
from NET formation. We also considered that the objects might not represent
apoptotic cells, given that apoptotic cells contain condensed DNA enclosed in
membrane, which is not observed in the objects. We thus suggest that the
hypothermia/rewarming-induced DNA unfolding is regulated in a manner distinct
from either canonical NETosis or canonical apoptosis, arguing the existence of
a previously unappreciated signaling pathway that alters global genomic DNA
structures in eukaryotic cells. Further, the results indicate that
coldtreatment followed by warming may affect NET formation, which is an
important consideration because many researchers use hypothermal conditions
during the isolation and culture of neutrophils. 5. Acknowledgements We thank
all the members of the Saitoh Laboratory for helpful discussion. This work was
supported by research grant to H. S. from Astellas Foundation for Research on
Metabolic Disorders, and by intramural founding in Kumamoto Health Science University
to J. K.